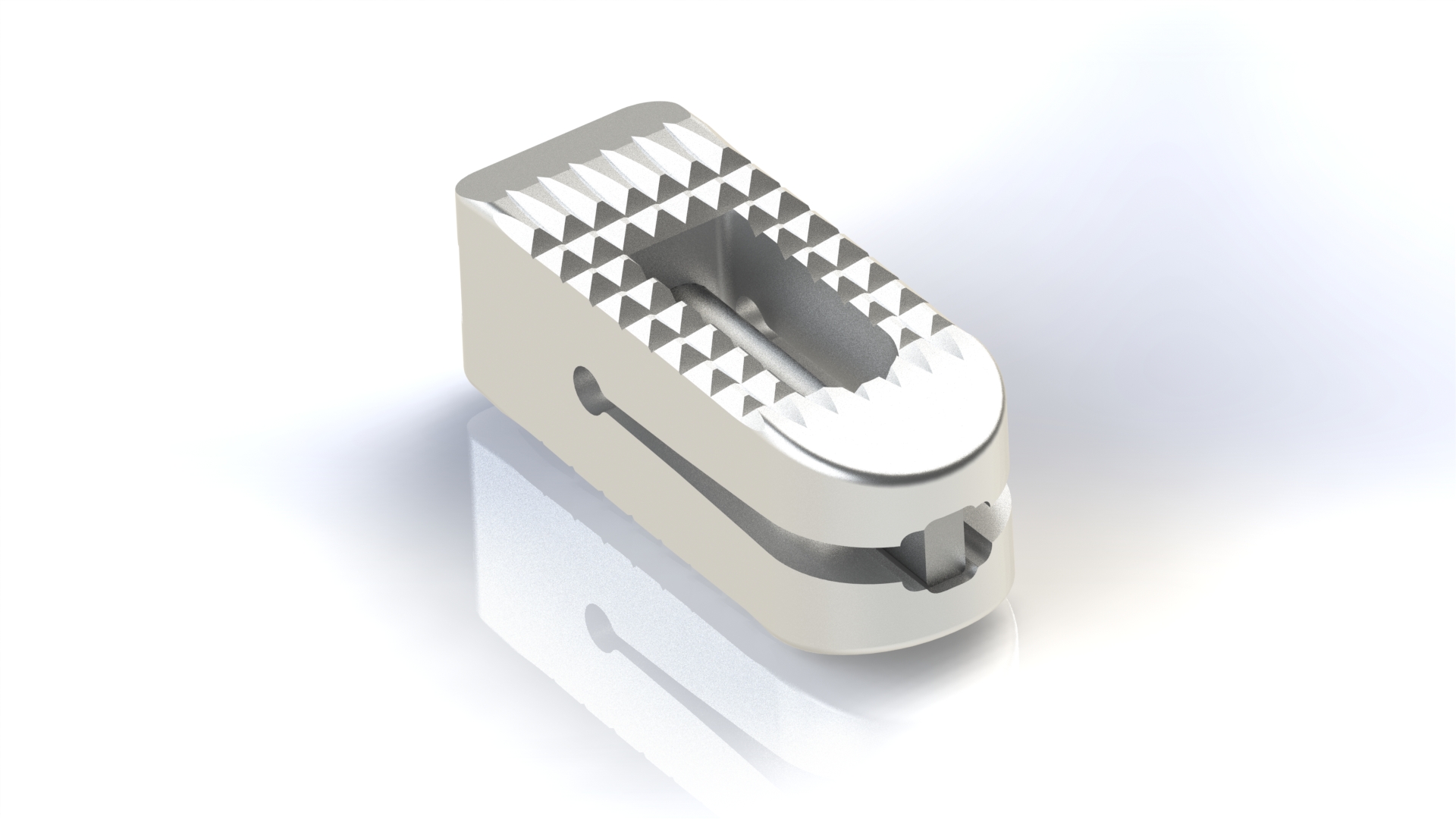
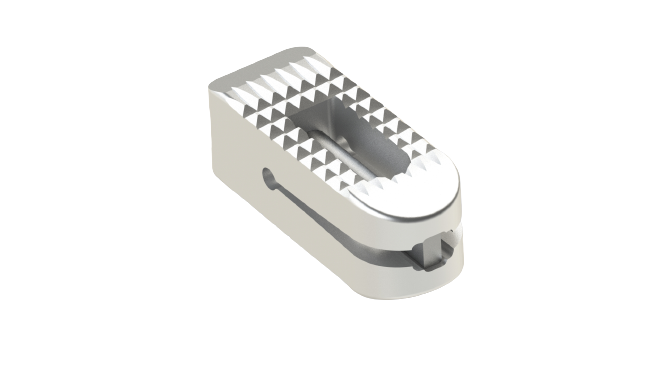
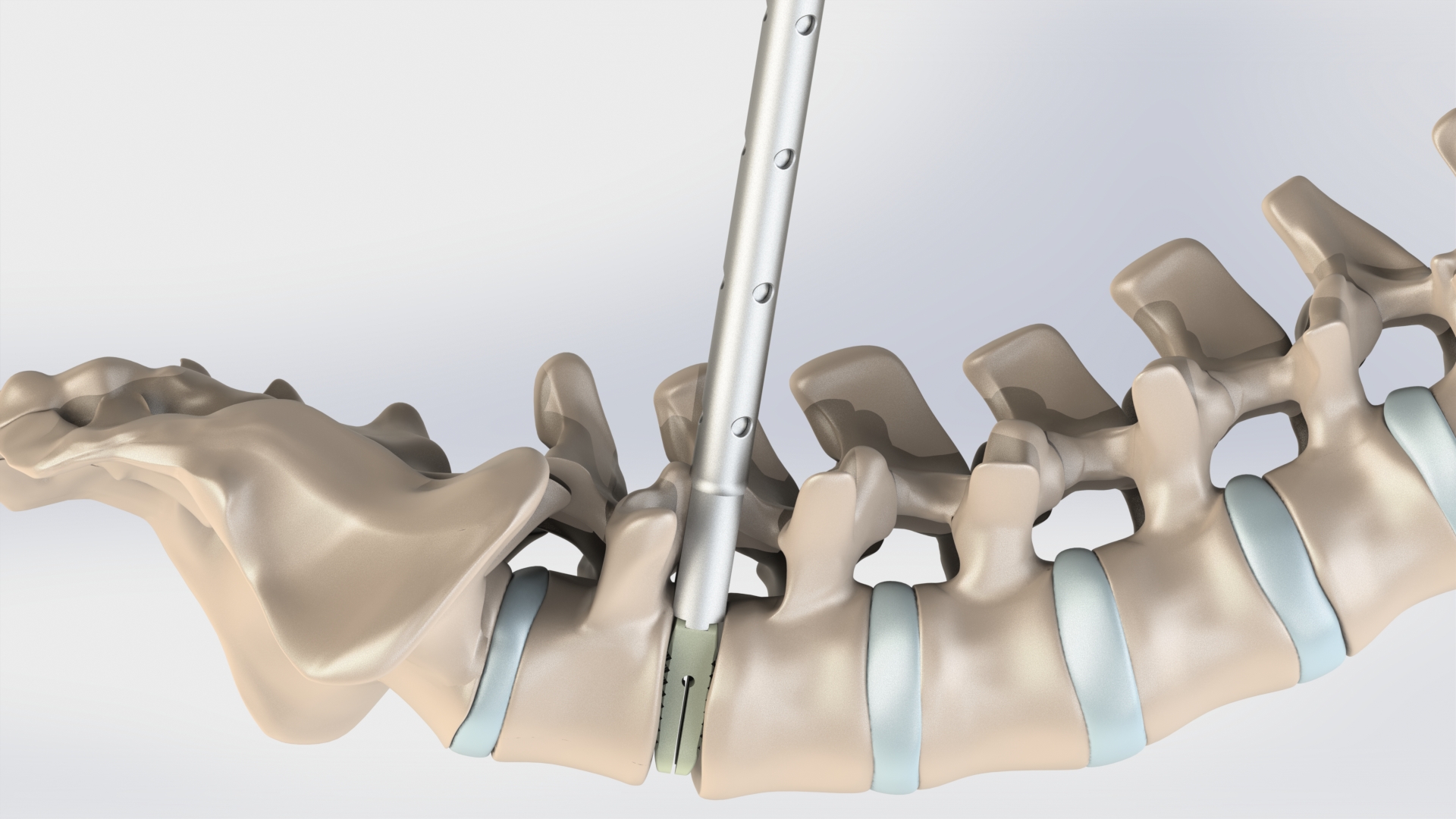
LorX® Expandable PLIF Titanium Cage is a posterior intrabody fusion device used for correction and stabilization of the lumbo-sacral region, aimed at the treatment of lumbar and/or lumbo-sacral spine (L1–S1) diseases.
The LorX® Expandable PLIF Titanium Cage is designed to be used twice per level.
The LorX® Expandable PLIF Titanium Cage is designed to be used with auxiliary spinal fixation systems (posterior screw-rod systems, anterior plate systems, anterior screw-rod systems, etc.) and is not recommended for use alone.
The LorX® Expandable PLIF Titanium Cage can be used in adults who meet the indications and have adequate spinal strength.
INDICATIONS
The system is indicated for degenerated disc disease, spinal instability, degenerate spondylolisthesis, pseudoarthrosis, degenerate scoliosis and adult deformity.
CONTRAINDICATIONS
Contraindications – but not limited to – are listed below:
- Pathological obesity, pregnancy, significant osteoporosis, open wounds, severe local inflammation, dependency on pharmaceutical drugs, drug abuse or alcoholism, mental illnesses, significant osteopenia, known or suspected allergy or intolerance to implant material (foreign body sensitivity to the implant material), acute or chronic infections, lack of patient cooperation.
Tria Spine
WE SERVE LIFE
Certification
Tria Spine® and products has international certificates of CE, ISO 9001, ISO 13485: 2016. In addition to international certificates including USA approval.
Contact UsPenetration
Tria Spine® headquarters in Ankara / Although found in Turkey; Tria Spine® operates in 24 countries all over the world.
Contact UsInvestment
2021 - 9 New Product Range Planning to Launch 2022 and 2 New Product Range Planning to Launch
Contact Us