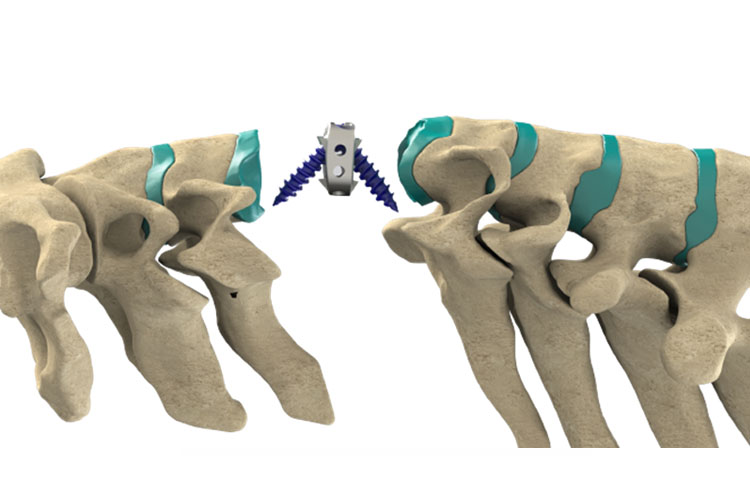
- Titanium: (Ti6Al4V ELI) Alloy
- Better stability
- With anchors and screws
- Safe
- Advanced design
- Anatomical shape
- Biocompatibility
- More fusion area
- With back lock screw
- Ready to sale also with bone greft (CE-DoC listed)
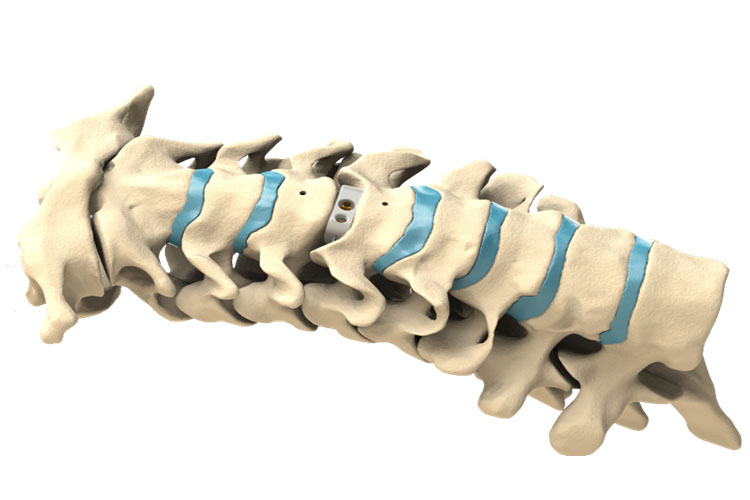
LorX® Cervical Titanium Cage is an anterior in-body fusion device for stabilization of the cervical region which aimed at the treatment of cervical spine diseases. The system is indicated for adult patients who has the spine at a maximum of 3 levels following discectomy for forced radioculopathy and / or myelopathy.
LorX® Cervical Titanium Cage is suitable for adolescents and adults who have enough bone stability and meet the main indications
INDICATIONS
The system is indicated for cervical degenerated disc disease, cervical disc hernia, cervical canal stenosis, and cervical kyphosis.
CONTRAINDICATIONS
Contraindications include but are not limited to:
Any medical or surgical condition that prevents the potential benefits of spine surgery, pathological obesity, pregnancy, overwhelming osteoporosis, open wounds, extreme local inflammation, drug and / or alcohol addiction, mental illness, extreme old age, body sensitivity to implant material, and / or allergy that preclude the potential benefits of spine surgery , acute or chronic infections, lack of patient cooperation.
Tria Spine
WE SERVE LIFE
Certification
Tria Spine® and products has international certificates of CE, ISO 9001, ISO 13485: 2016. In addition to international certificates including USA approval.
Contact UsPenetration
Tria Spine® headquarters in Ankara / Although found in Turkey; Tria Spine® operates in 24 countries all over the world.
Contact UsInvestment
2021 - 9 New Product Range Planning to Launch 2022 and 2 New Product Range Planning to Launch
Contact Us