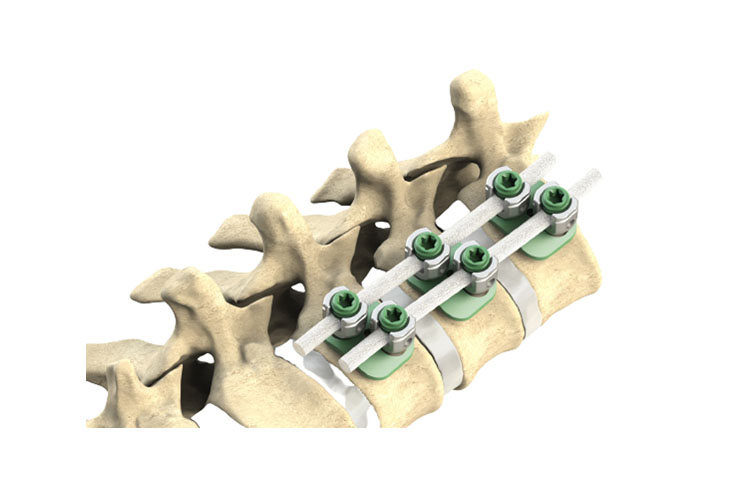
- Non-Fusion System
- Suitable for MIS Approach
- Cord: Polyethylene - Terephthalate
- Titanium: (Ti6Al4V ELI) alloy
- Cannulated and Non-Cannulated body design
- Better stability
- Biocompatibility
- Reliable solution for Idiopathic Scoliosis
KYRA® Correction Cord System is a fixing system which includes various types and sizes of pedicle screws, cord and nut.
KYRA® Correction Cord System is the medical device which treats scoliosis while a person is actively growing and uses their own growths to repair their curves.
KYRA® Correction Cord System is a spine device which has been designed for the treatment of idiopathic scoliosis.
The KYRA® Correction Cord System is different than typical scoliosis surgery which is called "spine fusion", which involves placing rigid metal bars on both sides of the spine to correct undesirable curves.
KYRA® Correction Cord System uses a strong, flexible cable to apply pulls outside a scoliosis curve to straighten of the spine instead of metal rods. The system uses the growth process to permanently straighten the spine.
Fixation systems are indicated for skeletally immature patients who require surgical treatment to achieve and maintain the correction of progressive idiopathic scoliosis. It is not suitable for use by adults.
INDICATIONS
KYRA® Correction Cord System which it is with a large Cobb angle of 30 to 65 degrees, as determined by radiographic imaging, the bony structure is dimensionally sufficient for screw fixation, it is indicated for skeletally immature patients who require surgical treatment to achieve and maintain progressive idiopathic scoliosis. It is not suitable for use by adults.
CONTRAINDICATIONS
The system is not available to use in the following situations below ; however, contraindications can not be limited to these.
- Systemic infection in surgical zone local infection or skin compromise at the surgical zone.
- Previous spine surgery at the levels which can be treated,
- As known poor bone quality defined as a T score of -1.5 or less
- Skeletal maturity
Other medical or surgical conditions that would block the potential benefit of spinal surgery, such as coagulation disorders, allergies to implant materials, and the inability to cooperate with patients post-operative care instructions.
Tria Spine
WE SERVE LIFE
Certification
Tria Spine® and products has international certificates of CE, ISO 9001, ISO 13485: 2016. In addition to international certificates including USA approval.
Contact UsPenetration
Tria Spine® headquarters in Ankara / Although found in Turkey; Tria Spine® operates in 24 countries all over the world.
Contact UsInvestment
2021 - 9 New Product Range Planning to Launch 2022 and 2 New Product Range Planning to Launch
Contact Us